Abstract
Introduction The drug-resistant mechanisms of the first-generation Bruton's tyrosine kinase (BTK) inhibitor, ibrutinib, has been extensively explored in chronic lymphocytic leukemia (CLL) patients. However, the resistant mechanisms of the second-generation BTK inhibitor such as zanubrutinib remained largely unexplored. We integrated multi-omics to assess the clonal evolutionary characteristics in CLL patients with zanubrutinib resistance.
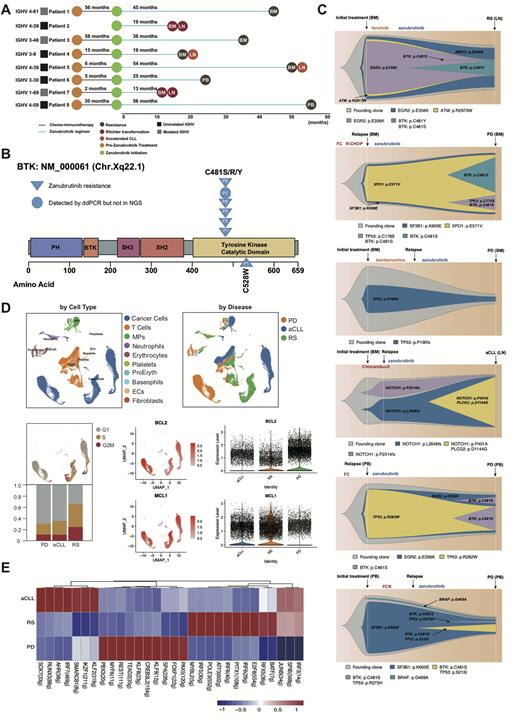
Methods We retrospectively identified 8 CLL patients with zanubrutinib resistance (1 first-line and 7 relapsed & refractory). Deep targeted-gene next generation sequencing (NGS) covering BTK (exon 1-19), PLCG2 (exon 1-33) and high sensitivity droplet digital PCR (ddPCR) detecting BTK Cys481 mutation were assessed in available serial samples. Single-cell RNA sequencing (scRNA-seq) of matching peripheral blood (PB) and lymph node (LN) were performed in 3 zanubrutinib-resistant patients showing progressive lymphadenopathy.
Results Totally 8 patients were included in our study, the median time from zanubrutinib initiation to progression was 30.5 months, including 2 patients were both histologically confirmed as Richter transformation (RT) after treatment of 13 months. 5 patients presented as LN enlargrment at progression received PET-CT imaging scan and further underwent LN biopsy or puncture at the site of highest SUVmax, 2 patients were diagnosed as RT, 2 as accelerated CLL (aCLL) and 1 as CLL progression. Deep targeted-gene NGS showing that BTK Cys481 mutation was detected in 5 of 8 patients (1 patients also harbored BTK Leu528Trp mutation with low frequency) and PLCG2 mutation was detected in one patient without BTK Cys481 mutation. For 3 patients with undetectable BTK mutation by NGS, ddPCR were performed and one patient was identified harboring BTK Cys481Ser mutation (progressed due to lymphadenopathy but NGS performed in bone marrow), suggesting spatial clonal heterogeneity in zanubrutinib-resistant patients.
Six patients were available for longitudinal deep targeted-gene NGS and the dynamic model of clonal evolution were depicted during zanubrutinib resistance. TP53, EGR2, NOTCH1 and SF3B1 were main driving clones in zanubrutinib resistance. 2 patients showed new-emerging TP53 mutation clones at the time of progression and another 2 patients showed persistent stable TP53 mutation clones. SF3B1, EGR2 and BIRC3 mutation clones maintained its stability during zanubrutinib resistanc while BTK Cys481, as the secondary drug resistance mechanisms, evolved during zanubrutinib treatment and underwent clonal expansion due to positive clone selection.
For 3 patients showing progressive lymphadenopathy, matched PB and LN scRNA-seq were performed. Notably, highest proportion of proliferating tumor cells was found in RT patients. The analysis of BCL-2 anti-apoptotic family genes expression showed that tumor cells in RT showed significantly higher expression of MCL-1 while tumor cells in progressive CLL showed relatively higher expression of BCL-2, indicating the shift of BCL-2 family dependence among different disease characteristics. Transcriptional regulon analysis showed that transcriptional factors like PAX5, FOXP1 and KLF8 displayed higher activity in progressive CLL and factors like ATF3, IRF5 and IRF8 showed relatively higher activity in RT. The transcriptional regulation of BCL-2 anti-apoptotic family genes expression remained to be explored further.
Conclusion: Integrated multi-omics were performed in our zanubrutinib-resistant CLL patients cohort. Due to spatial heterogeneity and clonal evolution among patients, deep targeted-gene NGS and ddPCR should be used complementarily to evaluate the emergence of resistant clones. BTK Cys481 and Leu528 were two main BTK resistant mutations in zanubrutinib resistant CLL patients. Moreover, TP53, EGR2, NOTCH1 and SF3B1 were also found remain persistent and be the driving clones during zanubrutinib resistance. scRNA-seq analysis of BCL-2 anti-apototic family expression revealed that CLL patients progressed to RT depended mainly on MCL-1 while CLL progressive patients depended on BCL-2, indicating that higher MCL-1 expression of RT might lead to insensitivity to venetoclax treatment following zanubrutinib resistance.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.